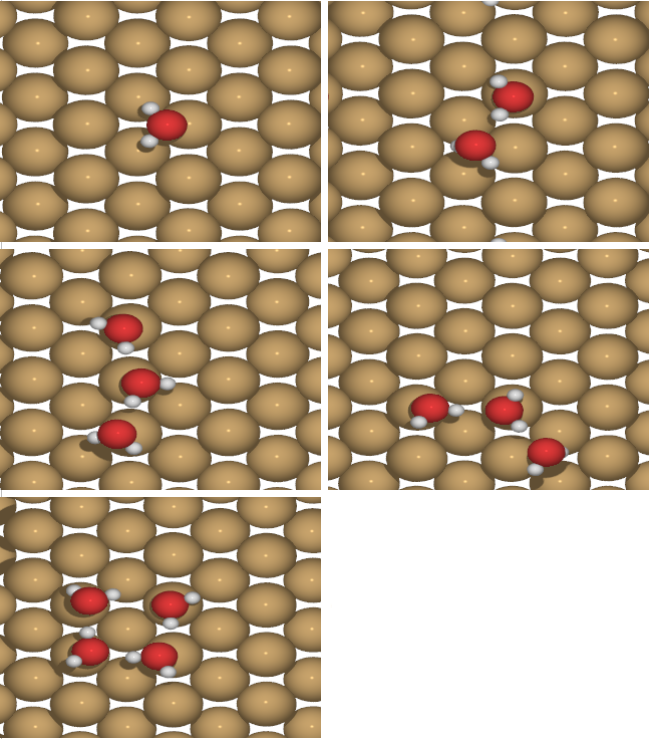
Figure 1 Clusters of water molecule. Adsorption energy increases by increasing the size of cluster
Position Indication:
Content

2011 - Amirreza Baghbanpourasl
Thermodynamics of adsorption of H2O on Cu(110) surface
Dissertationsprojekt: Research at Institute for Theoretical Physics, University of Paderborn
01.02.2011 – 31.04.2011
Amirreza BAGHBANPOURASL
Contact: vorname.nachname(/\t)jku.at
In collaboration with K. Hingerl (JKU Linz) and S. Wippermann and W.G. Schmidt (University of Paderborn) this research stay lead to the following results:
Introduction
Adsorption of Water molecule on copper surface has been the subject of plenty of studies. Theoretical investigations are done without considering the effect of environment. Here we investigate copper surface in thermodynamic equilibrium with environment and take into account chemical potential of Water molecules and Hydroxyl. the later is dependent on the acidity (pH value) of the system in contact with surface.
In this study we calculate phase diagram of surface structures with respect to relevant environment parameters which are chemical potential of water molecule and H+ ions.
Methods
Simulation
water clusters and overlays are investigated:
Water clusters: monomer, dimer, trimer, tetramer, pentamer
1 Mono layer: adlayers with intact water molecules , adlayers with water molecules and Hydroxyl
For each cluster size we have different configurations, the configuration with highest adsorption energy is the most stable one.
![]()
Here n is the number of water molecules. EH2O is the energy of water molecule at gas state. Etotal is the energy of super cell after adsorption and Ebare is the energy of the bare surface.
To be able to compare the systems with different number of water molecules and also for being able to consider the effect of environment on the structure, we have to calculate Grand Canonical potential Ώ
![]()
Here G is Gibb's free energy and i goes over the number of species that are H2O and OH. For our condensed system we can use the internal energy at T=0 calculated by DFT assuming PV term is very small and entropy term cancels out in different configurations.
![]()
Results and Discussion
Clusters up to pentamers were simulated. Stable geometry and adsorption energy were calculated for each cluster size.
Several full overlays also were simulated. The most stable one among intact overlays (in which full water molecules are adsorbed are calculated)
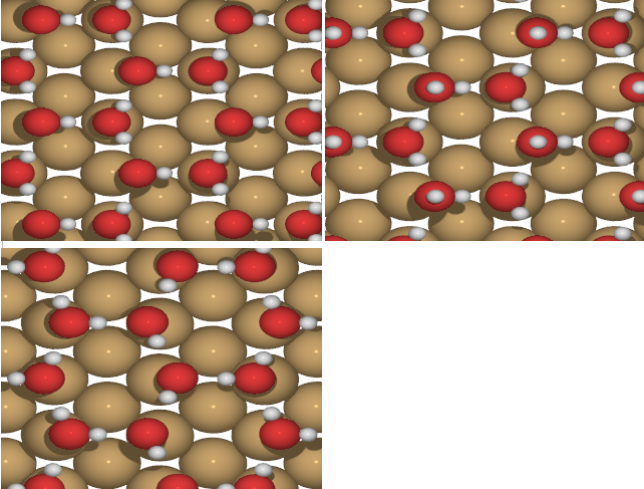
Figure 2 Intact water molecule monolayer. Most stable one is H_down (top left)
Overlays with partially dissociated water molecules are also simulated.
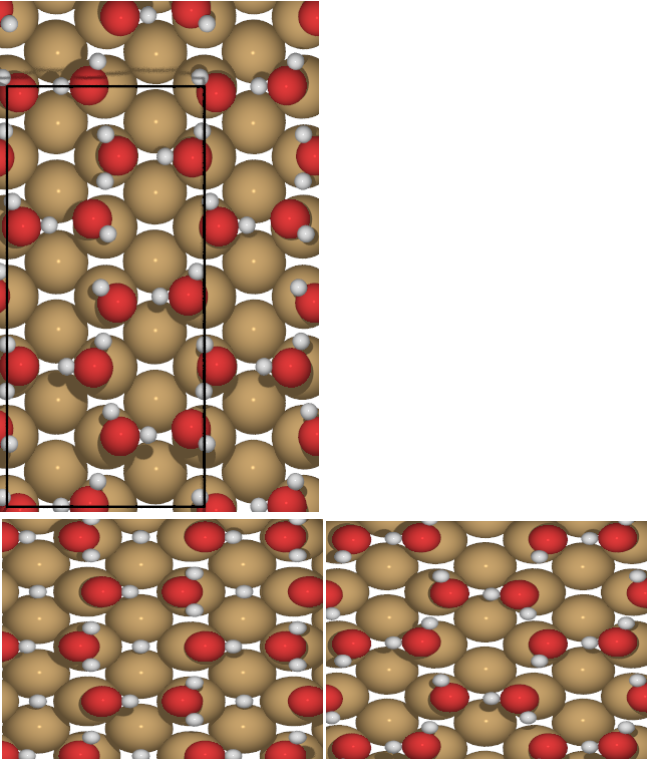
Figure 3 Partially dissociated water molecule monolayer: PDO2 (top), PDO1 (bottom right)
For calculating the phase diagram we should calculate grand canonical potential. For each predicted stable structure we calculate this potential using the resulted ground state energy.
This method can take into account different coverages of the same configuration.
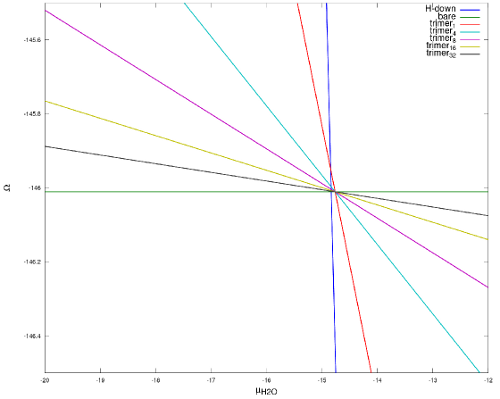
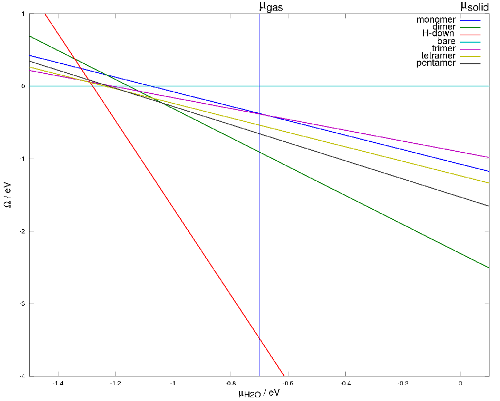
Phase diagram of water molecule of intact structures is only a function of one variable that is chemical potential of water molecule. This phase diagram (Figure 5) shows that in thermodynamic equilibrium, among all intact structures non of clusters will appear.
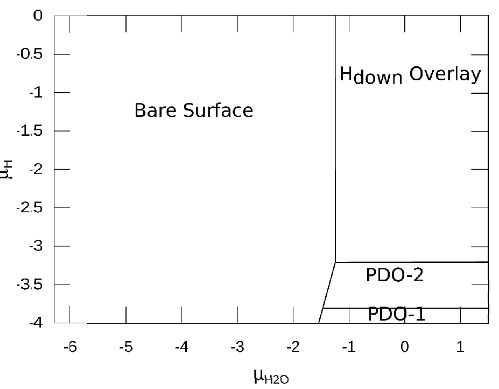
Figure 6: two dimensional phase diagram
Here general form of phase diagram has 2 arguments: chemical potential of water and chemical potential of H+.
The calculated phase diagram reasonably predicts the equilibrium state structures. By increasing the abundance of water molecules, full coverage appear and by decreasing the abundance of H+ ions, structures with higher ratio of Hydroxyl appear.
Conclusion
The calculated phase diagram, reasonably predicts formation of structures in different environmental conditions. This approach can predict formation of structures in different experimental conditions.
Bibliography
[1] J. Ren, S. Meng, PRB 77, 054110 (2008)
[2] M. Forster, et. al., PRL 106, 046103 (2011)
[3] T. Kumagai, et. al., The Journal of Chemical Physics 134, 024703 (2011)
[4] G.-X. Qian, et. al. PRL 60, 1962–1965 (1988)


 Audit hochschule und familie
Audit hochschule und familie