fig. 1 DBDP-C3, 7.14*7.14nm, E= 279mV, I= 0.1nA
Position Indication:
Content

2011 - Gholamreza Barati
Surface Physics at the liquid / Solid Interface
Research stay at Institute of Physical and Theoretical Chemistry (PTC) of University of Bonn, Germany
Gholamreza Barati
Contact: vorname.nachname(/\t)jku.at
From July 2010 to May 2011, I worked at the institute of Physical and theoretical chemistry (PTC) of University of Bonn for being trained with their home-made electrochemical scanning tunneling microscope (ECSTM) and for learning electrochemical approaches of using ECSTM together with Cyclic Voltammetry (CV) methods in the pertinent field of their research, that is self assembly of organic molecules on metal surfaces.
Electrochemistry is important for industries concerned with processes occurring in batteries, corrosion inhibition, electroplating. Since during electrochemical reactions electrons transfer occurs on the interface of electrode/solution, the mechanism of charge transferring is to be understood. There are different techniques to monitor the solid/liquid interfaces, from which the reflectance difference spectroscopy (RDS), spectroscopic ellipsometry(SE) and second harmonic and sum frequency generation(SHG & SFG) are of our consideration at ZONA. But also ECSTM is one of the main techniques to image the surface at atomic resolution, pioneered by K. Itaya(1) in Japan and K. Wandelt(2) in Bonn, Germany.
Under the supervision of Prof Wandelt I studied for a year the chloride modified copper (100) surface as a well-defined substrate for self assembly of some organic molecules which are of interest in nowadays surface science researches. Different kind of organic molecules were already studied by Prof Wandelt’s group on copper and gold single crystalline surfaces (3). Therefore as first steps towards working with ECSTM, measuring the chloride modified copper (100) surface, imaging the Dibenzyl viologen(DBV), Diphenyl viologen(DPV) structures at different potentials for copper as working electrode(WE) versus reversible hydrogen electrode(RHE) as reference electrode were accomplished that results were represented by the first report to this committee.
In the later stage of our measurements in a cooperative work with M. Saracino, we measured a new structure of molecules Dibenzyl dipridinum(DBDP-C3) which has a long chain 3 methylene group between the pridinum rings with the same pridinium/benzyl- half molecule structure of DBV. DBV has two different meta-stable structures on Cl/Cu(100) which is due to its change of redox behavior as mono- and di-cationic phases i.e. DBV+1 and DBV+2 . DBV+1 makes long-range ordered stripes at negative potentials while by increasing the potential to more positive ones it forms the so called “cavitand phase” composed of DBV+2 assemblies appears to the STM image(3). Despite of the similarity between DBV and DBDP-C3, we expected the same redox behavior. However, as XPS measurements at BESSY in proved DBDP-C3 reacts in the whole potential window as di-cations. Our results of imaging DBDP-C3 on Cl/Cu(100) are shown in fig. 1 & 2. Fig. 1 has been taken at a bias potential of 279mV and tunneling current of 0.1nA, while in fig (2) the potential and tunneling current have been set (after one third of the scan) deliberately and rather fast to the mentioned values in the caption of fig. 2. Then clearly the DBDP-C3 and the chlorine layer beneath were imaged simultaneously in one picture. The first analyses reveal that the periodic bright dots with a separation of 1 to 1.2 nm are nitrogen molecules capable of a charge transfer to the STM tip. Further analyses are needed to define the structural arrangements of molecules on the substrate. Final results will be published in collaboration with PTC Bonn, Germany.
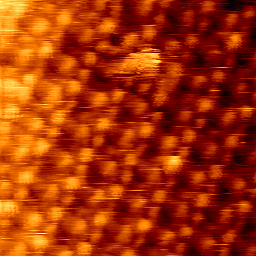
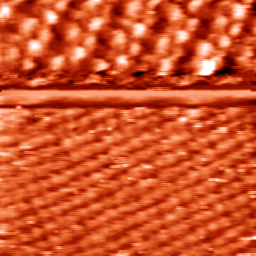
fig. 2 DBDP-C3, 7.14*7.14nm, E=110mV, I= 5nA
After returning to Linz, the experiment of Bonn was set up at a ZONA lab in the basement of the TNF tower. This ECSTM setup at ZONA is shown in fig. 3.
As outlook in Linz we will investigate Ag(110) and Cu(110) single crystalline surfaces with ECSTM and also with optical techniques.
References
[1] “In situ scanning tunneling microscopy in electrolyte solutions”, K. Itaya, Prog. Surf. Sci. 58, 3, 121 (1998)
[2] “A new and sophisticated electrochemical scanning tunneling microscope design for the investigation of potentiodynamic processes”, M. Wilms, M. Kruft, G. Bermes, and K. Wandelt, Rev. Sci. Instrum. 70, 3641 (1999)
[3] Cited in http://www.chemie.uni-bonn.de/pctc/wandelt/publikation-1/publikationen-1


 Audit hochschule und familie
Audit hochschule und familie