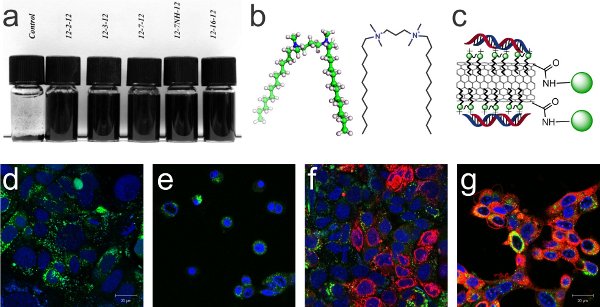
Figure 1: (a) SWNT dispersion in gemini surfactants; series of cationic (N,N-bis(dimethylalkyl)-α2,ω-alkanediammonium) (m-s-m) gemini surfactants with varying spacer group exhibiting a high capacity to disperse SWNTs, (b) 3D structure and schematic representation of the 12-3-12 gemini surfactant molecule. (c) Schematic representation of CNT complex for gene delivery to cells; oxidized SWNT complexed with gemini surfactant molecules, electrostatically attached nucleic acids and covalent binding of quantum dots as fluorescent reporter. (d-g) Confocal microscopy images of PAM212 keratinocyte cells for comparison of siRNA delivery and toxicity of applied treatment after 24 h with DRAQ5 nuclear stain in blue and calcein cell viability dye in green ; (d) no treatment, (e) 0.1% 12-3-12 gemini surfactant (toxic), (f) pure siGLO-RNA (red), (g) complexes of 0.1% 12-3-12 gemini surfactant and siGLO-RNA (red) have improved transfection efficiency compared to pure siGLO-RNA and reduced toxicity compared to pure Gemini surfactant. We expect further improvement using the complex depicted in (c).
Position Indication:
Content

2011 - Constanze Lamprecht
Carbon nanotubes for transdermal and ocular gene-delivery
PostDoc Project: School of Pharmacy, University of Waterloo, Ontario, Canada
2 - 3 years, start date: 15.03.2011
Constanze Lamprecht
Contact: vorname.nachname (at) gmx.de
In the past two decades, the discovery of carbon nanotubes (CNTs) has opened new frontiers in the field of nanotechnology and nanoscience which inevitably lead to their intersection with biology and medicine. Though, the application of CNTs in the biomedical field is significantly hampered by their inherent hydrophobic nature, significant progress in chemical modification and functionalization has paved the way towards their use in aqueous media. Most of the functionalization schemes fall into one of the two main categories: a) covalent and b) non-covalent functionalization of the outer CNT surface. In covalent functionalization schemes, CNTs are usually subjected to a rigorous chemical treatment to generate reactive species that improve aqueous solubility. Whereas non-covalent dispersion procedures make use of amphiphilic molecules that act as surfactants where the hydrophobic tail adsorbs to the CNT surface via non-covalent interactions including π-π stacking, van der Waals and charge transfer, while the hydrophilic part of the molecules imparts aqueous solubility. Having nanoscale dimensions, water soluble CNTs can cross biological barriers, such as cell membranes. I addition, their high surface area facilitates attachment of multiple copies of different chemical and biological entities. With this combination CNTs possess huge potential as multipurpose drug delivery and diagnostic systems in nanomedicine.
The aim of my PhD research at JKU in the Institute of Biophysics was to establish a more detailed understanding of cellular uptake of bio-functionalized CNTs as an important premise for future medical applications. My studies focused on the binding of functionalized carbon nanotubes to cells and their delivery into the cell on a single molecule level using atomic force microscopy and fluorescence microscopy. In the Foldvari research group at the School of Pharmacy of the University of Waterloo I will continue my work on CNT based drug-delivery towards the exploitation of CNTs as actual pharmaceutical excipients. Our overall objective is to develop new strategies and pharmaceutical products using CNT as excipients for transdermal and ocular delivery of therapeutics, a field that is little explored at the current state. The Wilhelm Macke Mobility Scholarship (funding period: 6 months) provides me with the necessary funding to implement my research plan at the Foldvari Lab and start first experiments. This initial funding will further help and enable me to prepare for a grant application at Canadian and international funding bodies to fully support my research at the University of Waterloo.
The first stage of the project involves the design and biophysical characterization of novel approaches for CNT bio-functionalization, and subsequently their application for gene delivery. We propose the use of a new class of surfactants as CNT solubilizers (Figure 1a,b). The so called gemini surfactants consist of two identical hydrophobic chains and two hydrophilic moieties connected through a spacer group (Figure 1b). They have attractive properties, such as low critical micelle concentration (CMC) and a high surface activity which essentially reduces the amount of surfactant required to disperse CNTs. Through variations in the hydrophobic chain-length, the hydrophilic head groups, and the spacer group, a wide range of tailored compounds can be designed . Moreover, cationic gemini molecules were found to form stable complexes with nucleic acids and improve gene transfection in vitro, leading to the successful expression of therapeutic proteins (Figure 1d-g). With CNTs as a multimodal carrier and gemini surfactant as a tunable surface coating that shows great potential for gene therapies, we have a potentially capable platform for a range of different gene therapy applications (Figure 1c).
Prof. Marianna Foldvari and her research team have shown that geminis have a superior ability for dispersing CNTs compared to other pharmaceutical surfactants (Figure 1a) - patent applications pending . For our envisaged project we have started to identify and assess CNT materials from different commercial sources for their suitability for incorporation into pharmaceutical products and formulations . In parallel we are evaluating the effect of CNT dimensions on the delivery of siRNA/gemini surfactant/multi-walled carbon nanotube complexes into keratinocytes, a type of human skin cells. For this study we are using CNTs with varying outer diameters but similar lengths . In addition we will determine the influence of electronic type (semiconducting, metallic) of single walled carbon nanotubes on cell transfection. These experiments and resulting publications will provide the foundation for upcoming grant applications for funding of the project on “Application of CNTs for transdermal and ocular gene delivery”.
References
[1] Liu Z, Tabakman S, Welsher K, Dai HJ 2009 Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery Nano Research 2 85-120
[2] Menger FM, Keiper JS 2000 Gemini surfactants Angew Chem Int Edit 39 1907-20
[3] Hait SK, Moulik SP 2002 Gemini surfactants: A distinct class of self-assembling molecules Curr Sci India 82 1101-11
[4] Wettig SD, Verrall RE, Foldvari M 2008 Gemini surfactants: A new family of building blocks for non-viral gene delivery systems Curr Gene Ther 8 9-23
[5] Badea I, Verrall R, Baca-Estrada M, Tikoo S, Rosenberg A, Kumar P, et al. 2005 In vivo cutaneous interferon-gamma gene delivery using novel dicationic (gemini) surfactant-plasmid complexes J Gene Med 7 1200-14
[6] Ivanova MV, Loureiro MJ, Lamprecht C, Foldvari M 2011 Non-covalent functionalization of carbon nanotubes with gemini surfactants for pharmaceutical applications Manuscript in preparation
[7] Lamprecht C, Huzil JT, Ivanova MV, Foldvari M 2011 Non-covalent functionalization of carbon nanotubes with surfactants for pharmaceutical applications – A critical mini-review Drug Delivery Letters 1 in press
[8] Foldvari M, Verrall RE, Badea I, inventors; Dna Delivery with Gemini Cationic Surfactants 2008.
[9] Foldvari M, inventor DISPERSION AND DEBUNDLING OF CARBON NANOTUBES USING GEMINI SURFACTANT COMPOUNDS patent WO 2010055416 (A1) 2010.
[10] Foldvari M. Formulating nanomedicines: Focus on carbon nanotubes as novel nanoexcipients. In: Vallet-Regí M, Vila M, editors. Key Engineering Materials: Trans Tech Publications, Switzerland; 2010. p. 53-74.
[11] Ivanova MV, Loureiro MJ, Lamprecht C, Foldvari M 2011 Pharmaceutical characterization of commercial solid and dispersed carbon nanotube materials Manuscript in preparation
[12] Saliaj E, Elsabahy M, Lamprecht C, Foldvari M 2011 Size dependent delivery of siRNA into keratinocytes using different diameter multi-wall carbon nanotubes Manuscript in preparation
[13] Saliaj E, Elsabahy M, Ivanova MV, Foldvari M. Confocal microscopic study of diameter dependent delivery of siRNA into keratinocytes by multiwall carbon nanotubes. NT10 11th International Conference on the Science and Application of Nanotubes; June 2010; Montreal, Canada2010.


 Audit hochschule und familie
Audit hochschule und familie